Authors:
Kimberly J. Felcher, Michigan State University
David Douches, Michigan State University
Objective
- Demonstrate the use of a marker for the H1 locus to select for resistance to golden nematode in a conventional potato breeding program.
Introduction
For a new cultivar to be successful, it must have an advantage over existing varieties that can be readily observed and jointly agreed upon by both growers and processors (for processing varieties) and by growers and consumers (for fresh market and novelty varieties). For breeders, this means not only selecting for numerous traits, but combining those traits into one variety. Another challenge for breeders is to be able to identify and breed for traits before they are demanded by growers, processors, and consumers. Therefore, the goal of most potato breeding programs is twofold: develop improved varieties and increase the frequency of important alleles in the program germplasm.
Approximately 85% of the potatoes grown in Michigan are utilized by the potato chip industry. As a result, the potato breeding program at Michigan State University is dedicated to developing new potato chip varieties. Currently, the emphasis is on varieties that meet or exceed current agronomic standards, resist cold-sweetening, produce acceptable chips with low acrylamide levels, and have scab (Streptomyces scabies) resistance. Additional traits such as resistance to late blight (Phytophthora infestans), potato virus Y (PVY), Verticillium wilt (Verticillium dahlia), and golden nematode (Globodera rostochiensis) are being incorporated into early generation breeding materials. Although these additional traits are not presently required by growers, processors, or consumers, and are not currently viewed as market limiting traits in Michigan, they represent potential challenges to our potato industry and may already be challenges in other regions of potato production.
Golden nematode (GN) is a soil-borne, potato cyst nematode (PCN) that is a concern in some US potato growing regions and has been recognized in Europe as a significant pest of potatoes for many years. Heavy infestations of this pest can cause significant yield losses and limit the choice of potato varieties that can be grown effectively. The first reported identification of GN in the United States was in New York in 1941. Subsequent Federal and State quarantines have limited the spread of this pest to 9 counties in New York. In addition to limiting the spread of this pest, the best management practices include: rotating fields with non-host crops (corn, soybeans and wheat) and growing GN resistant potato varieties (Animal and Plant Health Inspection Service [APHIS], 2008).
Phenotypic screening for golden nematode resistance
Phenotypic screening for GN resistance is done in replicated greenhouse tests. Briefly, tubers are planted in pots and inoculated with G. rostochiensis eggs. Eight weeks after emergence, the plants are uprooted and the number of cysts are counted (0-5 = resistant; >5 = susceptible) (Brodie and Plaisted, 1987). The time and cost involved in this assay are prohibitive to screening thousands of early generation breeding lines for GN resistance. Therefore, when limited to phenotypic screening, only a small number of later generation breeding lines can be screened for GN resistance based on a pedigree suggestive of the potential for resistance. An alternative to phenotypic screening is marker-assisted selection (MAS).
Genotypic screening for golden nematode resistance
Several GN resistance loci and quantitative trait loci (QTL) have been identified in wild potato species (Table 1). The H1 locus from S. tuberosum ssp. andigena has been widely used in potato breeding programs since its discovery in 1953 (Toxopeus and Huijsman, 1953) and can be found in many GN resistant potato varieties. In addition, a high-density map of the region surrounding the H1 locus has been constructed, making it possible to develop markers and conduct MAS for this locus (Bakker et al., 2004). A PCR-based marker tightly linked to the H1 locus was developed (W. De Jong, personal communication) and is being used successfully to select for GN-resistant breeding material. Primers and experimental conditions are listed in Table 2.
| Gene | Chromosome | G. rostochiensis Pathotype | Source | Reference |
|---|---|---|---|---|
| Gro1 Gene Family | VII | Ro1 | S. spegazzinii | Barone et al. (1990), Ballvora et al. (1995) |
| Gro1-4 | VII | Ro1 | S. spegazzinii | Paal et al. (2004) |
| Gro1.2 (QTL) | X | Ro1 | S. spegazzinii | Kreike et al. (1993) |
| Gro1.3 (QTL) | XI | Ro1 | S. spegazzinii | Kreike et al. (1993) |
| Gro1.4 (QTL) | III | Ro1 | S. spegazzinii | Kreike et al. (1996) |
| GroVI | V | Ro1 | S. vernei | Jacobs et al. (1996) |
| H1 | V | Ro1,Ro4 | S. tuberosum ssp. andigena | Bakker et al. (2004), Toxopeus and Huijsman (1953) |
| Primer Name | Primer Sequence | Cycling Profile | Diagnostic Band (bp) |
|---|---|---|---|
| TG689-F | TAA AAC TCT TGG TTA TAG CCT AT |
94°C, 2 min. 35 cycles: 94°C, 20s; 55°C, 20s; 72°C, 30s 72°C, 5 min. |
H1 marker = 141 BCH control = 290 |
| TG689-R | CAA TAG AAT GTG TTG TTT CAC CAA | ||
| BCH-F | CAT GAC ATA GTT TGA ATT TTG AGT C | ||
| BCH-R | CGT TTG GCG CTG CCG TAA GTT |
MAS for H1-based golden nematode resistance
In the Michigan State University Potato Breeding and Genetics program, MAS for golden nematode resistance is conducted after the 3rd year of a breeding cycle. At harvest, the third-year (12-hill) plots are evaluated and approximately 25% of the lines (roughly 500) are selected for advancement. Of the selected lines, those with the potential (based on pedigree) to harbor the H1 resistance locus are genotyped. For genotyping, template DNA is extracted from leaf tissue that is collected from the plants growing in the field or from post-harvest tuber tissue. A high-throughput, 96-well DNA extraction protocol is used to increase the efficiency and cost effectiveness of the process. Following PCR, using the template DNA and the H1 locus primers, and electrophoretic separation on an agarose gel (2%), the individual clones are scored for the presence or absence of the 119 bp band linked to the H1 locus. Breeding lines that are positive for the H1 marker band are suspected to be resistant to GN (Fig. 1). Although market-limiting traits are given priority, the presence of the H1 marker band helps us make more informed decisions during future rounds of selection. Furthermore, lines that have the H1 marker but are not selected to advance towards commercialization are often used as parents for subsequent rounds of crossing and selection. This increases the frequency of this valuable allele in the program germplasm. Breeding lines that are being considered for commercialization and test positive for the H1 marker are phenotyped to ensure that they are GN resistant.
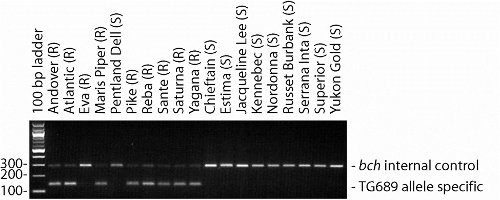
Figure 1. Results of PCR amplification of the H1 locus from several commercial potato varieties. Varieties followed by (R) are GN resistant based on phenotypic evidence, those followed by (S) are susceptible. Almost all resistant varieties exhibit the TG689 allele-specific band (141 bp); known exceptions include the resistant varieties ‘Eva’, ‘Salem’, and ‘Sunrise’. The 290 bp band is an internal control to verify that the PCR reaction worked and should be present in all varieties. Photo credit: Dan Zarka, Michigan State University Potato Breeding and Genetics Program.
Conclusions
Although GN is not a current problem for Michigan potato growers, it is still important to develop GN-resistant potato varieties that meet the needs of Michigan growers. By doing so, we ensure that if GN ever becomes a problem in our state, acceptable potato varieties will be available to avoid losses due to this pest. Utilizing MAS to select for the H1 locus makes our breeding program more efficient and increases the probability that future breeding lines will be GN resistant.
References Cited
- Animal and Plant Health Inspection Service—United States Department of Agriculture. 2008. Fact sheet: Golden nematode [Online]. USDA. Available at: http://www.aphis.usda.gov/publications/plant_health/content/printable_version/fs_golden_nematode_08.pdf (verified 1 Mar 2012).
- Ballvora, A., J. Hesselbach, J. Niewöhner, D. Leister, F. Salamini, and C. Gebhardt. 1995. Marker enrichment and high-resolution map of the segment of potato chromosome VII harbouring the nematode resistance gene Gro1. Molecular and General Genetics 249: 82–90. (Available online at: http://dx.doi.org/10.1007/BF00290239) (verified 1 Mar 2012).
- Bakker, E., U. Achenback, J. Bakker, J. van Vliet, J. Peleman, B. Segers, S. van der Heijden, P. van der Linde, R. Graveland, R. Hutten, H. van Eck, E. Coppoolse, E. van der Vossen, J. Bakker, and A. Goverse. 2004. A high resolution map of the H1 locus harbouring resistance to the potato cyst nematode Globodera rostochiensis. Theoretical and Applied Genetics 109: 146–152. (Available online at: http://dx.doi.org/10.1007/s00122-004-1606-z) (verified 1 Mar 2012).
- Barone, A., E. Ritter, U. Schachtschabel, T. Debener, F. Salamini, and C. Gebhardt. 1990. Localization by restriction fragment length polymorphism mapping in potato of a major dominant gene conferring resistance to the potato cyst nematode Globodera rostochiensis. Molecular and General Genetics 224: 177–182. (Available online at: http://dx.doi.org/10.1007/BF00271550) (verified 1 Mar 2012).
- Brodie, B. B., and R. L. Plaisted. 1987. Resistance in the potato cv. Highlat Russet to golden nematode, Globodera rostochiensis. American Journal of Potato Research 64: 523–524. (Available online at: http://dx.doi.org/10.1007/BF02853721) (verified 1 Mar 2012).
- Jacobs, J.M.E., H. J. van Eck, K. Horsman, P.F.P. Arens, B. Verker-Bakker, E. Jacobsen, A. Pereira and W.J. Stiekema. 1996. Mapping of resistance to the potato cyst nematode Globodera rostochiensis from the wild potato species Solanum vernei. Molecular Breeding 2: 51–60. (Available online at: http://dx.doi.org/10.1007/BF00171351) (verified 1 Mar 2012).
- Kreike, C. M., J.R.A. DeKoning, J. H. Vinke, J. W, Van Ooijen, and W. J. Stiekema. 1993. Mapping of loci involved in quantitatively inherited resistance to the potato cyst-nematode Globodera rostochiensis pathotype Ro1. Theoretical and Applied Genetics 87: 464–470. (Available online at: http://dx.doi.org/10.1007/BF00215092) (verified 1 Mar 2012).
- Kreike, C. M., A. A. Kok-Westeneng, and J. H. Vinke. 1996. Mapping of QTLs involved in nematode resistsance, tuber yield and root development in Solanum sp. Theoretical and Applied 92: 463–470. (Available online at: http://dx.doi.org/10.1007/BF00223694) (verified 1 Mar 2012).
- Paal, J., H. Henselewski, J. Muth, K. Meksem, C. Menendez, F. Salamini, S. Ballvora, and C. Gebhardt. 2004. Molecular cloning of the potato Gro1-4 gene conferring resistance to pathotype Ro1 of the root cyst nematode Globodera rostochiensis, based on a candidate gene approach. Plant Journal 38: 285–297. (Available online at: http://dx.doi.org/10.1111/j.1365-313X.2004.02047.x) (verified 1 Mar 2012).
- Toxopeus, H. J., and C. A. Huijsman. 1953. Breeding for resistance to potato root eelworm. Euphytica 2: 180–186. (Available online at: http://dx.doi.org/10.1007/BF00053725) (verified 1 Mar 2012).
External Links
- Sowokinos, J. 2001. Biochemical and molecular control of cold-induced sweetening in potatoes. American Journal of Potato Research 78: 221–236. (Available online at: http://dx.doi.org/10.1007/BF02883548) (verified 1 Mar 2012).
- Wikipedia contributors. 2010. Acrylamide. Wikipedia, The Free Encyclopedia. Available at: http://en.wikipedia.org/w/index.php?title=Acrylamide&oldid=395749979. (verifed 1 Mar 2012).
- Wharton, P. S. 2010. Tuber diseases [Online]. Michigan Potato Diseases. Michigan State University. Available at: http://www.potatodiseases.org/tuberdiseases.html (verified 1 Mar 2012).
- Wharton, P. S. 2010. Foliar diseases [Online]. Michigan Potato Diseases. Michigan State University. Available at: http://www.potatodiseases.org/foliardiseases.html (verified 1 Mar 2012).
- Johnson, S. B. 1995. Verticillium wilt of potatoes. Bulletin #5041. Potato Facts. University of Maine Cooperative Extension. Available at: http://www.umaine.edu/umext/potatoprogram/Fact%20Sheets/verticillium%20wilt.pdf (verified 1 Mar 2012).
- Wang, X., and S. Gray. 2006. Potato cyst nematodes – Pests of national importance [online]. USDA-ARS Cornell University. Available at: http://www.ars.usda.gov/Main/docs.htm?docid=12126 (verified 27 Mar 2012).
- Wikipedia contributors. 2010. Marker assisted selection. Wikipedia, The Free Encyclopedia. Available at: http://en.wikipedia.org/w/index.php?title=Marker_assisted_selection&oldid=396502815. (verified 1 Mar 2012).
Funding Statement
Development of this page was supported in part by the National Institute of Food and Agriculture (NIFA) Solanaceae Coordinated Agricultural Project, agreement 2009-85606-05673, administered by Michigan State University. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the United States Department of Agriculture.
PBGworks 908
