Authors:
Carlos Hernandez-Garcia, The Ohio State University; Zhifen Zhang, The Ohio State University
The Genetics of Pepper Heat: Significant Genes Identified in Capsaicinoid Biosynthesis
Pungency is a unique characteristic of pepper caused by a group of alkaloids called capsaicinoids. In nature, it is hypothesized that pepper plants use capsaicinoids to deter frugivorous (fruit-eating) mammals from consuming their fruits. However, birds are not affected by pungency as they may not have capsaicin receptors. As a result, birds not only feed on pepper fruits but also disperse pepper seeds efficiently (Tewksbury and Nabhan, 2001). Humans, on the other hand, can enjoy the heat from pepper and spices derived from pepper via the papillae, located on the tongue. Particularly, humans can sense capsaicinoids via a receptor structurally related to members of the TRP family of ion channels. Interestingly, this receptor is also activated by increases in temperature, suggesting that it functions as a transducer of painful thermal stimuli in vivo. Similar to other receptors important in sensory neurons, the capsaicin receptor reversibly loses sensitivity to capsaicin as well as other pain and heat stimuli when it is under prolonged exposure to the same stimulus (Caterina et al., 1997; Winter et al., 1995). This phenomenon may partially explain why humans can tolerate and even enjoy spicy foods, as pain sensors may be turned off. Capsaicin has also had wide usage as a pain reliever in medicine. All the aforementioned features make peppers one of the most important vegetables produced globally.
Capsaicinoid biosynthesis and accumulation, exclusive to the Capsicum genus, are genetically determined in pepper fruits, but they are also developmentally and environmentally regulated. The Capsicum genus includes over 20 species of peppers, from which C. annuum, C. frutescens, C. chinense, C. baccatum, and C. pubescens are domesticated (Walsh and Hoot, 2001). There is wide genetic variation in pungency level across Capsicum species. For example, the non-pungent sweet bell pepper from C. annuum scored 0 SHU (Scoville Heat Unit; a scale that indicates the amount of capsaicin), while ‘Bhut Jolokia’, a putative interspecific hybrid between C. chinense and C. frutescens from Northeastern India, scored up to 1,001,304 SHUs (Bosland and Baral, 2007). The latter is currently considered the hottest pepper in the world, even hotter than the ‘Red Savina’ Habanero (up to 577,000 SHUs). This high genetic-based variation in pungency level provides a foundation to breed chili peppers with different amounts or types of capsaicinoids.
Capsaicinoids are composed of an aromatic moiety derived from vanillylamine and an acyl group, as a result of the convergence of the phenylpropanoid and the branched-chain fatty acid synthesis pathways. Starting from the phenylpropanoid pathway, vanillylamine is produced after a series of steps. At last, vanillyamine is transformed into an amide (capsaicinoid) after the vanillyamine reacts with an acyl group contained in a fatty acid. Therefore, enzymes involved in vanillylamine synthesis and fatty acid synthesis are important for capsaicinoid synthesis. Variation in the structure of both the aromatic group and acyl group has been reported; however, the structure of the acyl groups, in particular, determines the intensity of pungency (Mazourek, et al., 2009). Although more than ten different capsaicinoid structures exist in chili pepper, capsaicin is the most abundant.
The capsaicinoid synthesis pathway could be the target of pepper pungency engineering and breeding because pungency intensity depends on capsaicinoid levels and their chemical structure. However, genetic control of capsaicinoid levels in different Capsicum species and varieties remains poorly understood. Although 55 candidate genes have been proposed to be involved in capsaicinoid synthesis (Mazourek et al., 2009), the Pun1 gene is the only gene identified to date as having significant effect on capsaicin levels. Pun1 is a dominant gene mapped to chromosome 2 (Blum et al., 2002; Stewart et al., 2005). Two recessive alleles, pun1 and pun12, conferring non-pungency, have also been identified and described (Stewart et al., 2005, 2007). Pun1 encodes an acyltransferase 3 (AT3), and its expression is up-regulated in the early fruit development stages (~20 days post-anthesis). Expression of this gene decreases ~40 days post-anthesis (Stewart et al., 2005). Molecular characterization of the pun1 gene has shown that a 2.5-kb deletion comprising the promoter and the first exon significantly reduces AT3 protein synthesis. Moreover, a 4-bp deletion located in the first exon of allele pun12 leads to a frame shift mutation resulting in an aberrant AT3 protein and therefore reduced levels of capsaicin. These genetic disruptions can reduce capsaicin levels up to 70% (Stewart et al., 2005).
Histological studies have revealed that capsaicinoids are accumulated in specialized structures named blisters, which are formed along the interlocular septum of pepper fruits. Formation of blisters is controlled by a recessive gene (lov), which was previously considered a second significant gene in capsaicin synthesis (Votava and Bosland, 2002). However, lov co-segregated with the pun12 allele, and they turned out to be the same allele of the Pun1 gene (Stewart et al., 2007). A SNP located at the 3′ end of the Pun1 gene was also identified between pungent and non-pungent peppers (Garces-Claver et al., 2007). This SNP has been proposed as a marker for selection of pungency and non-pungency traits.
With the available plant genomes, we performed a rapid analysis for the presence of the Pun1 gene in different plants. A BLAST search of the Pun1 gene sequence (Genbank entry: GU300812.1) revealed no complete genes homologous to the Pun1 in tobacco and tomato (Fig. 1). However, we observed DNA fragments with high similarity to this gene but lying on different contigs, which suggests translocation of segments of the Pun1 gene in these two plants. Translocation of specific gene fragments may have affected the function of this gene, thereby limiting pungency to the Capsicum genus. It is also possible that the different gene fragments observed in the tobacco and tomato genomes correspond to homologous acyltransferases such as AT1 and AT2 (Stewart Jr et al., 2005). The analysis of the Pun1 gene sequence in poplar, Arabidopsis, and soybean did not identify any Pun1 homologues (Fig. 1).
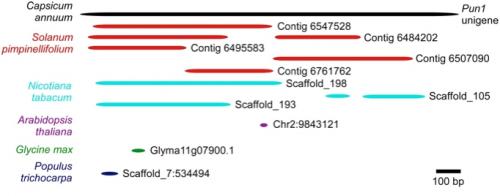
Figure 1. Alignment of DNA fragments from different plant genomes and with high similarities to the Pun1 acyltransferase unigene (Genbank entry: GU300812.1). Bars for each species represent homologous regions to the Pun1 gene sequence. Figure credit: Carlos Hernandez-Garcia, The Ohio State University.
In addition to Pun1 on chromosome 2, two major quantitative trait loci (QTLs) playing an important role in capsaicin production have been identified on chromosome 7. These QTLs strongly interact with QTL fw2, located on chromosome 2. Fw2 plays a role in determining fruit weight (Blum et al., 2003; Ben-Chaim et al., 2006). The interaction between QTLs on chromosome 7 and 2 explained 37-42% of the variation in capsaicin contents, while the QTLs on chromosome 7 alone explained only 16-17% of the variation in capsaicin (Ben-Chaim et al., 2006). Moreover, fw2 is linked to the Pun1 locus (Ben-Chaim et al., 2006), confirming the importance of Pun1 in the capsaicinoid pathway. Two to three minor QTLs responsible for capsaicin and dihydrocapsaicin have also been identified on chromosomes 3 and 4. Candidate genes important in capsaicin synthesis have also been mapped to chromosomes 1, 2, 3, 4, 6, 7, 8, 11, and 12. The chromosomes where these genes localize correspond to previous QTL mapping results (Table 1).
| Chromosome | Gene and QTLs | Candidate genes |
| 1 | Pun1, fw2.1 | Kas1 (3-xooacyl-[acyl-carrier-protein] synthase), Acl (acyl carrier protein) |
| 2 | Kas1 | |
| 3 | fw3.1, cap3.1 | Kas1, pAMT(aminotransferase) |
| 4 | cap4 (1, 2), dhc4 (1, 2) | BCAT (branched-chain amino-acid aminotransferase) |
| 6 | FatA (acyl-ACP thioesterase), Ca4H (cinnamic caffeic acid-3-O-methyltransferase) | |
| 7 | ndhc7, cap7 (1, 2), dhc7 (1, 2) | pAMT, 4A1 (acylCoA transferase) |
| 8 | Kas1, Acl | |
| 11 | 4A1 | |
| 12 | Kas1 |
Although the capsaicin biosynthesis pathway has been established, its genes and enzymes have been poorly characterized. A better understanding of capsaicinoid regulation and synthesis in pepper may be achieved by integrating both biochemistry and genetic approaches. The recent release of genome sequences for some Solanaceae and the use of genome-wide transcript profiling such as RNAseq will surely facilitate the elucidation of the genes forming the capsaicinoid pathway. A full understanding of this trait is imperative not only to develop new pepper varieties with modified levels of pungency but also to alter capsaicin levels in existing varieties.
References Cited
- Ben-Chaim, A., Y. Borovsky, M. Falise, M. Mazourek, B. Kang, I. Paran, and M. Jahn. 2006. QTL analysis for capsaicinoid content in Capsicum. Theoretical and Applied Genetics 113: 1481–1490. (Available online at: http://dx.doi.org/10.1007/s00122-006-0395-y) (verified 11 Jan 2011).
- Blum, E., K. Liu, M. Mazourek, E. Y. Yoo, M. Jahn, and I. Paran. 2002. Molecular mapping of the C locus for presence of pungency in Capsicum. Genome 45: 702–705. (Available online at: http://dx.doi.org/10.1139/G02-031) (verified 11 Jan 2011).
- Blum, E., M. Mazourek, M. O’Connell, J. Curry, T. Thorup, K. D. Liu, M. Jahn, and I. Paran. 2003. Molecular mapping of capsaicinoid biosynthesis genes and quantitative trait loci analysis for capsaicinoid content in Capsicum. Theoretical and Applied Genetics 108: 79–86. (Available online at: http://dx.doi.org/10.1007/s00122-003-1405-y) (verified 11 Jan 2011).
- Bosland, P. W. and J. B. Baral. 2007. ‘Bhut jolokia’ – the world’s hottest known chile pepper is a putative naturally occurring interspecific hybrid. HortScience 42: 222–224. (Available online at: http://hortsci.ashspublications.org/cgi/content/abstract/42/2/222) (verified 11 Jan 2011).
- Caterina, M.J., M. A. Schumacher, M. Tominaga, T. A. Rosen, J. D. Levine, and D. Julius. 1997. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 389: 816–824. (Available online at: http://dx.doi.org/10.1038/39807) (verified 11 Jan 2011).
- Garces-Claver, A., S. M. Fellman, R. Gil-Ortega, M. Jahn, and M. S. Arnedo-Andres. 2007. Identification, validation and survey of a single nucleotide polymorphism (SNP) associated with pungency in Capsicum spp. Theoretical and Applied Genetics 115: 907–916. (Available onilne at: http://dx.doi.org/10.1007/s00122-007-0617-y) (verified 11 Jan 2011).
- Iezzoni, A. 2010. Jewels in the Genome. RosBREED Newsletter 1(1): 9. (Available online at: http://rosbreed.s3.amazonaws.com/wp-content/uploads/2010/10/2010-02_RosBREED.Newsletter.pdf) (verified 13 Jan 2011).
- Mazourek, M., A. Pujar, Y. Borovsky, I. Paran, L. Mueller, and M. M. Jahn. 2009. A dynamic interface for capsaicinoid systems biology. Plant Physiology 150: 1806–1821. (Available online at: http://dx.doi.org/10.1104/pp.109.136549) (verified 11 Jan 2011).
- Stewart, C., B. C. Kang, K. Liu, M. Mazourek, S. L. Moore, E. Y. Yoo, B. D. Kim, I. Paran, and M. M. Jahn. 2005. The Pun1 gene for pungency in pepper encodes a putative acyltransferase. Plant Journal 42: 675–688. (Available online at: http://dx.doi.org/10.1111/j.1365-313X.2005.02410.x) (verified 11 Jan 2011).
- Stewart, C., Jr., M. Mazourek, G. M. Stellari, M. O’Connell and M. Jahn. 2007. Genetic control of pungency in C. chinense via the Pun1 locus. Journal of Experimental Botany 58: 979–991. (Available online at: http://dx.doi.org/10.1093/jxb/erl243) (verified 11 Jan 2011).
- Tewksbury, J. J. and G. P. Nabhan. 2001. Seed dispersal – directed deterrence by capsaicin in chillies. Nature 412: 403–404. (Available online at: http://dx.doi.org/10.1038/35086653) (verified 11 Jan 2011).
- Votava, E. J. and P. W. Bosland. 2002. Novel sources of non-pungency in Capsicum species. Capsicum & Eggplant Newsletter 21: 66–68.
- Walsh, B. M. and S. B. Hoot. 2001. Phylogenetic relationships of capsicum (solanaceae) using DNA sequences from two noncoding regions: The chloroplast atpB-rbcL spacer region and nuclear waxy introns. International Journal of Plant Sciences 162: 1409–1418. (Available online at: http://dx.doi.org/10.1086/323273) (verified 13 Jan 2011).
- Winter, J., S. Bevan and E. A. Campbell. 1995. Capsaicin and pain mechanisms. British Journal of Anaesthesia 75: 157–168.
Additional Resources
The Chili Pepper Institute http://www.chilepepperinstitute.org/
Acknowledgements
The authors thank Drs. David Francis and Heather Merk for critically reading this review and providing helpful suggestions.
Funding Statement
Development of this lesson was supported in part by the National Institute of Food and Agriculture (NIFA) Solanaceae Coordinated Agricultural Project, agreement 2009-85606-05673, administered by Michigan State University and CONACYT, Mexico. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the United States Department of Agriculture or any of the other aforementioned entities.
PBGworks 930
